Author: Jorge E. Ginorio
Institution: University of Connecticut
Date: October 2006
Abstract
Polylactide can be processed into fibers and films for various biomedical applications, including sutures, dialysis media, and drug delivery devices. In this work, well-defined biodegradable polylactide (PLA) containing block copolymers, poly(ethylene-co-1,2-butylene)-b-poly(D-lactide) (PEB-b-PDLA) and poly(ethylene oxide)-b-poly(L-lactide) (PEO-b-PLLA), were synthesized via ring-opening polymerization of D- and L-lactides initiated from PEB-OH and PEO-OH macroinitiators, respectively. The goal of this research was to obtain stereocomplexes from PLA block copolymers containing highly immiscible blocks such as hydrophilic PEO and hydrophobic PEB. The molecular weight and molecular weight distribution (< 1.16) of purified block copolymers were characterized by 1H NMR and size-exclusion chromatography. PLA stereocomplex crystals were grown from the melt of an equimolar blend of PEB-b-PDLA and PEO-b-PLLA and had a single melting peak at 199 °C, indicative of a quantitative formation of stereocomplexes. This was further confirmed by wide-angle X-ray diffraction (WAXD) experiments. The morphology of these stereocomplexes was investigated by small-angle X-ray scattering.
Introduction
Polylactide (PLA) is a biodegradable, thermoplastic, aliphatic polyester. Due to the chiral nature of the lactide monomers, several distinct forms of polylactide exist, such as poly(L-lactide) (PLLA), poly(D-lactide) (PDLA), and poly (D,L-lactide) [P(DL)LA or simply PLA]. Polylactide can be processed, like most thermoplastic polymers, into fibers and films for various biomedical applications, including sutures, dialysis media, and drug delivery devices. PLA is also attractive as a sustainable alternative to petrochemical-derived products, since it can be produced from a more economic resource such as corn starch.
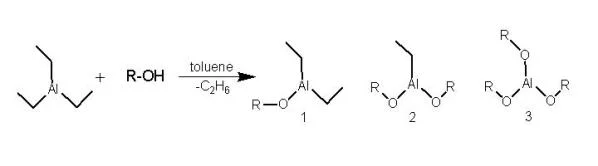
Block copolymers containing PLA blocks combine the properties of PLA and other polymers, and thus have found numerous practical usages. For example, poly(ethylene oxide)-b-PLA (PEO-b-PLA) is an amphiphilic block copolymer with PEO being hydrophilic and PLA being hydrophobic. The di- and tri-block copolymers from PEO and PLA are used as temperature sensitive injectable drug delivery systems (Jeong, 1997). PLA block copolymers can be synthesized through ring-opening polymerization (ROP) of lactides, initiated from hydroxyl-terminated macroinitiators using catalysts such as stannous octoate [Sn(Oct)2] in the melt (Kricheldorf, 2000). However, well-defined polymers with a narrow molecular weight distribution are difficult to obtain using Sn(Oct)2 catalyzed ROP. Instead, macro-initiators from reactions of triethyl aluminum (AlEt3) with hydroxyl end-functionalized polymers can initiate ROP of cyclic esters such as lactide and ε-caprolactone, resulting well-defined block copolymers (Schmidt, 1999 and Wang, 2000; see Figures 1 and 2).
article_819_order_1
article_819_order_2
A polymer stereocomplex is formed from the stereoselective interaction between two complementing stereoregular polymers, which interlock and form a new composite with altered physical properties in comparison to the parent polymers. (Slager, 2003) Since the first stereocomplex formation between enantiomeric PLAs (PLLA and PDLA) reported in 1987, numerous studies have been carried out on the formation, structure, property, degradation, and applications of PLA stereocomplexes (Tsuji, 2005). The formation of stereocomplex enhances mechanical properties, thermal-resistance, and hydrolysis-resistance of PLA based materials. These improvements arise from the strong interaction between the L- and D-lactyl unit sequences, and thus open a new way for the preparation of biomaterials such as hydrogels and particles for drug delivery systems.
Most studies on enantiomeric PLA block copolymer stereocomplexes focused on compatible pairs such as PEO-b-PDLA and PEO-b-PLLA (Lim, 2000). However, studies on PLA block copolymers with other incompatible blocks were rare in the literature (Kricheldorf, 2005). In this work, well-defined poly(ethylene-co-1,2-butylene)-b-PDLA (PEB-b-PDLA) and PEO-b-PLLA block copolymers with low polydispersity index [defined as the ratio of weight- to number-average molecular weights (Mw/Mn), PDI<1.16] were synthesized by ROP of D- and L-lactides, initiated from hydroxyl end-functionalized PEB and PEO using AlEt3 as the catalyst, respectively (see Figures 1 and 2). Since PEO is hydrophilic and PEB is hydrophobic, they are highly immiscible. This study attempts to understand the competition between the microphase separation of PEB and PEO and the stereocomplex formation of PDLA and PLLA at a 1:1 molar ratio. Differential scanning calorimetry (DSC) and small-angle X-ray scattering (SAXS) and wide-angle X-ray diffraction (WAXD) were employed to characterize both base copolymers and their stereocomplexes.
Materials and Methods
Materials
Poly(ethylene glycol) methyl ether (PEO-OH) with a Mn of 4,800 g/mol and poly(ethylene-co-1,2-butylene) mono-ol (PEB-OH) with a Mn = 4,000g/mol were purchased from Aldrich. AlEt3 (Aldrich, 1.9 M solution in toluene) was used as received. Dry toluene was distilled from calcium hydride, freeze-degassed, and stored under a dry nitrogen atmosphere.
Synthesis
The synthesis of macroinitiators was conducted in a glove box. PEB-OH (PEO-OH) was dissolved in toluene in a dry Schlenk flask. An aliquot of 1.9 M AlEt3 in toluene at a ratio of [OH]/[AlEt3] = 2.0 was slowly added to the flask using a syringe. The mixture was allowed to stir for 2 hrs at room temperature to form macroinitiators. After addition of lactide monomers, the Schlenk flask was sealed and immersed in an oil bath at 90-100 °C for 3-5 hrs. The final molecular weight of the PDLA or PLLA block was controlled by the monomer conversion, and monitored by 1H NMR recorded using a 500 MHz NMR (Bruker DMX 500) spectrometer (see Figure 3). The crude polymer products were purified by dissolving in chloroform and precipitated in methanol for PEB-b-PDLA and hexanes for PEO-b-PLLA to remove unreacted monomers. PEB-b-PDLA was further washed by hexane (or PEO-b-PLLA by methanol) twice to remove unreacted PEB (PEO) homopolymers.
Stereocomplexes Preparation
Stereocomplexes were prepared by blending the PEB-b-PDLA and PEO-b-PLLA block copolymers at a 1:1 molar ratio in chloroform. Films were cast at room temperature and dried in a vacuum oven at 50 °C for 2 days. The stereocomplex sample was heated to 250 °C (above the melting point, Tm) for 10 min before a slow cooling to room temperature.
Characterization
Size exclusion chromatography (SEC) was performed on a Viscotek GPCmax system, using THF as the mobile phase at a flow rate of 1.0 mL/min and polystyrene as standards. The phase transitions in pure block copolymers and their stereocomplexes were characterized by DSC on a TA Q100 instrument at 10 °C/min heating and cooling rates. Both SAXS and WAXD experiments were performed at Advanced Polymer Beamline (X27C) in the National Synchrotron Light Source, Brookhaven National Laboratory. The wavelength of the X-ray was 0.137 nm. The beam center and scattering vector q (q = 4πsinθ/λ, where θ is the half scattering angle and λ is the wavelength) were calibrated using silver behenate with the primary reflection peak at 1.076 nm-1.
Results
Molecular Weight Characterization by SEC and 1H NMR
The starting PEB-OH and PEO-OH have relatively low PDI of 1.04 and 1.08, respectively. The SEC results for the PEB-b-PDLA (4.0k-5.4k) and PEO-b-PLLA (4.8k-4.9k) are shown in Figure 4. Both block copolymers after purification had relatively low PDI, e.g., 1.09 for PEB-b-PDLA (4.0k-5.4k) and 1.14 for PEO-b-PLLA (4.8k-4.9k). Due to the conventional calibration used in the SEC experiments, the absolute molecular weights of the PDLA and PLLA blocks in the block copolymers could not be accurately obtained. However, they were determined by 1H NMR shown in Figure 3, using the molar ratios between PEB/PDLA and PEO/PLLA in the block copolymers.
Thermal Transitions Studied by DSC
article_819_order_3
DSC results of the phase transitions in PEB-b-PDLA (4.0k-5.4k), PEO-b-PLLA (4.8k-4.9k), and their stereocomplexes grown from the melt are shown in Figure 5. In Figure 5A, PEO and PLLA crystals had Tms around 47.3 °C and 143.7 °C in PEO-b-PLLA (4.8k-4.9k), respectively, while PDLA crystals had a Tm of 154.5 °C in PEB-b-PDLA (4.0k-5.4k). The slightly higher melting temperature of the PDLA crystals in PEB-b-PDLA could be attributed to the fact that PEB and PDLA were highly immiscible while PEO and PLLA were miscible in the melt. For the stereocomplex DSC in Figure 5B, a much higher Tm was found at 199.3 °C, and no Tm for either PDLA or PLLA crystals was observed at ~150 °C, indicating that stereocomplex was formed quantitatively. The PEO crystal Tm was observed at ca. 46 °C.
X-ray Diffraction Studies
article_819_order_4
Quantitative stereocomplex formation was also confirmed by WAXD shown in Figure 6B. For the PEO-b-PLLA, both reflections from PEO and PLLA crystals were clearly seen (e.g., PLLA (010)LA and (110)LA/(200)LA reflections, and PEO (120)EO and (032)EO reflections). However, the strong PLA (110)LA/(200)LA reflections disappeared in the WAXD profile of the stereocomplex. Instead, reflections from the stereocomplex crystals (i.e., (110)SC, (300)SC, and (220)SC (Cartier 1997)), were clearly seen, together with the PEO crystal reflection such as (120)EO. The SAXS profile of the stereocomplex grown from the melt is shown in Figure 6A. A broad peak was observed with an overall d-spacing at 33.2 nm. Note that this overall lamellar spacing was much longer than that of the PEO-b-PLLA (4.8k-4.9k) (~19.9 nm).
Discussion
article_819_order_5
The synthesis of macroinitiators and the subsequent ROP needs to be conducted in an inert environment free of oxygen and moisture because trace amount of oxygen or moisture will terminate the reaction. The increase in molecular weight needs to be monitored by carefully extracting aliquots from the reaction flask with a predetermined time interval and tested by 1H NMR. The 1H NMR and SEC results in Figures 3 and 4 indicate that well-defined polylactide block copolymers were obtained with narrow molecular weight distribution.
article_819_order_6
On the basis of both DSC (Figure 5B) and WAXD (Figure 6B) results, PLLA/PDLA stereocomplexes form with a quantitative yield, despite the strong immiscibility between the hydrophilic PEO and hydrophobic PEB blocks. In the stereocomplex PEO was crystalline with a melting point at 45 °C, suggesting that it microphase separate from hydrophobic PEB to form individual domains. The plausible morphology could be a PEO-Stereocomplex-PEB-Stereocomplex four-layer crystal shown in the schematic of Figure 6A.
Limitations
DSC study only gives information on phase transitions and transition temperatures. No morphological information can be obtained from a DSC study alone. WAXD is a powerful technique to identify crystal structures at sub-nanometer scales, while SAXS is used to reveal morphology such as the overall lamellar thickness at a few tens to 100 nanometers. No information on individual layer thickness is readily available for multicomponent systems. Therefore, detailed morphology in these stereocomplexes needs to be studied by transmission electron microscopy (TEM) technique, which is currently underway.
Conclusions
To obtain stereocomplexes from PLA block copolymers containing immiscible blocks, well-defined PEO-b-PLLA and PEB-b-PDLA diblock copolymers with a narrow molecular weight distribution (<1.16) were synthesized by ROP of the lactide monomers from PEO-OH and PEB-OH starting polymers using AlEt3 as the catalyst. The quantitative formation of stereocomplex from equimolar PEB-b-PDLA and PEO-b-PLLA blends was testified by DSC and WAXD studies, which also suggested a strong microphase separation between highly immiscible PEO and PEB blocks that were tethered to the stereocomplex crystals of PDLA and PLLA. These new stereocomplex materials may be useful in biomedical applications.
Acknowledgements
The author is indebted to the NSF REU Program at Polymer Program of University of Connecticut. The research was also supported by ACS PRF-G (41918-G7) and NSF Career Award (DMR-0348724). X-ray experiments were performed at National Synchrotron Light Source (NSLS), Brookhaven National Laboratory and supported by the Department of Energy.
References
Cartier, L., Okihara, T. and Lotz, B. (1997) Triangular polymer single crystals: Stereocomplexes, twins, and frustrated structures. Macromolecules 30, 6313-22.
Jeong, B., Bae, Y. H., Lee, D. S., and Kim, S. W. (1997) Biodegradable block copolymers as injectable drug-delivery systems. Nature 388, 860-862.
Kricheldorf, H. R., Kreiser-Saunders, I., and Stricker, A. (2000) Polylactones 48. SnOct2-initiated polymerizations of lactide: A mechanistic study. Macromolecules 33, 702-709.
Kricheldorf, H. R., Rost, S., Wutz, C., and Domb, A. (2005) Stereocomplexes of A-B-A triblock copolymers based on poly(L-lactide) and poly(D-lactide) A blocks. Macromolecules38, 7018-7025.
Lim, D. W. and Park, T. G. (2000) Stereocomplex formation between enantiomeric PLA-PEG-PLA triblock copolymers: Characterization and use as protein-delivery microparticulate carriers J. Appl. Polym. Sci. 75, 1615-1623.
Schmidt, S. C. and Hillmyer, M. A. (1999) Synthesis and charactherization of model polyisopropene-polylactide diblock copolymers. Macromolecules 32, 4794-4801.
Slager, J. and Domb, A. (2003) Bipolymer stereocomplexes. Adv. Drug Delivery Rev. 55, 549-583.
Tsuji, H. (2005) Poly(lactide) stereocomplexes: Formation, structure, properties, degradation, and applications. Macromol. Biosci. 5, 569-597.
Wang, Y. B. and Hillmyer, M. A. (2000) Synthesis of polybutadiene-polylactide diblock copolymers using aluminum alkoxide macroinitiators. Kinetics and mechanism.Macromolecules 33, 7395-7403.