Fahad Nadeem1, John Medley2, Sean Phan1, Haibin Ning3, Yongzhe Yan3, Aaron Catledge1*
1 Department of Physics, University of Alabama at Birmingham
2 Department of Chemistry, University of Alabama at Birmingham
3 Department of Mechanical and Materials Engineering, University of Alabama at Birmingham
Abstract
Injectable bone grafts represent a major market in bone regeneration due to their void filling capability and tendency for osteogenesis. Calcium phosphate is commonly used as a synthetic bone graft but is limited in mechanical strength, precluding its use in load-bearing clinical applications. As a potential solution, Engineered Cementitious Composites (ECCs) are being studied for their load-carrying capacity and energy absorption. The ECC model reported that ≤ 2% by volume of discontinuous fiber is favorable for strain-hardening behavior and enhanced flexibility in commercial concrete. Based on this model, this work developed injectable calcium phosphate bone graft cements reinforced with cellulose nanocrystals (CNC) along with gelatin fibers, which revealed an enhanced crosslinked fiber-bridging property across a matrix crack due to the strong intermolecular hydrogen bonding of cellulose and gelatin and their induced friction at the interface. We hypothesized that the fibrous reinforcement would increase the hardness and toughness of the bone matrix up to a critical CNC volume ≤ 2%, after which diminished mechanical properties were anticipated due to fiber aggregation and reduced hydrogen bonding. Therefore, bone grafts with varying CNC concentrations were synthesized in order to investigate the effect of fiber reinforcement on graft hardness and toughness, which was measured through standard mechanical testing apparatuses. The intermolecular hydrogen bonding and surface morphology was analyzed through Fourier-transform infrared (FTIR) spectroscopy and scanning electron microscopy (SEM). While the results did not display statistically significant increases in hardness or toughness with CNC reinforcement, the hardness significantly decreased after the 1–2% CNC critical point. This decrease is further supported by FTIR, which displayed decreased levels of cellulose-gelatin hydrogen bonding. SEM also showed that low-fiber CNC samples exhibited extensive microcracking compared to higher-fiber CNC samples, which contained larger cracks and fiber aggregates. These results imply that CNC reinforcement is not conducive to enhanced strain hardening behavior nor toughness below the critical volume fraction, and exceeding CNC concentrations are associated with decreased fiber bonding and matrix support which leads to fracture failure. The significance of these results imply that ECC reinforced with CNC may not be a suitable model for mechanically optimizing commercial bone grafts.
Author Summary
The Engineered Cementitious Composite (ECC) model has found that ≤ 2% by volume of discontinuous fiber is favorable for strain-hardening behavior and enhanced ductility in commercial concrete. Using this model, we attempted to mechanically optimize injectable calcium phosphate bone grafts via fiber reinforcement of cellulose nanocrystals (CNC) and gelatin polymer fibers. Results displayed significant decreases in the bone grafts’ hardness after the 1–2% CNC critical point (p < 0.001) due to fibril agglomeration, which likely prompted internal cracking and lowered the samples’ tolerance for plastic deformation. In support, Fourier transform infrared (FTIR) spectroscopy displayed decreased levels of cellulose-gelatin hydrogen bonding after this critical point due to decreases in transmittance broadening. Scanning electron microscopy (SEM) also showed that low-fiber CNC samples exhibited extensive microcracking compared to higher-fiber CNC samples, which contained larger cracks and fiber aggregates, indicating catastrophic failure and reduced hydrogen bonding between cellulose and gelatin.
Introduction
Bone Grafting Overview
As an organ, the bone is integral in providing support, mobility, and protection to the body. The properties of bone, such as strength and regeneration, are mainly attributed to collagen protein and the mineral crystals that form the bone tissue. While bones are strong, they are still very susceptible to damage through high-strength forces that can cause fractures or even diseases like osteoporosis (Bone health and osteoporosis: a report of the Surgeon General, 2004). Bone grafting is one of the most commonly used surgical methods to augment bone regeneration in orthopedic and dental procedures (Dimitriou et al., 2011). The bone grafts act as a bioabsorbable and mineral reservoir, which induces new bone formation. The goal of the replacement in the defect is to eliminate the dead space and help enhance the bone-healing. Bone grafting is possible because bone tissue is able to regenerate completely in the provided space in which it grows. As it grows and heals, the graft helps replace the dead space with a new bone region (Kumar et al., 2013).
There are three mechanisms that provide a rationale for bone grafting: osteoconduction, osteoinduction, and osteogenesis (Giannoudis et al., 2005). Osteoconduction occurs when the bone graft material serves as a scaffold for new bone, which is done through preserving the native bone (Kumar et al., 2013). Thus, an osteoconductive surface is one that permits bone grafts on its surface. Cells that form new bone tissue are called osteoblasts, which originate from bone marrow tissue (Laurencin et al., 2006). Osteoinduction involves the stimulation of cells that can differentiate into osteoblasts, which begins the formation of new bone (Kumar et al., 2013). Bone graft material that is both osteoconductive and osteoinductive not only serves as a scaffold for currently existing osteoblasts but also triggers the formation of new osteoblasts that can promote faster integration of the graft. Finally, osteogenesis occurs when the cells originating from the bone graft material contribute to the growth of new bone (Kumar et al., 2013).
Synthetic Bone Grafts Advantages and Clinical Use
Synthetic bone graft substitutes have evolved as alternatives to commercial orthopedic procedures such as allografts and autografts (Roberts et al., 2012). These bone graft substitutes prove to be viable alternatives to autogenous bone grafting as bone void fillers because they are more efficient, safer, inexpensive, available in different forms of pellets and injectable fluid, and are now common in use for different medical and dental applications, such as fractures, total joint replacements, and dental implants (Özcan et al., 2021). These materials mimic the minerals of the bone that are reabsorbed at a rate similar to bone formation (Tay et al., 1999). Osteoconductive and osteoinductive bone grafts have grafting factors such as bone morphogenetic proteins that help recruit mesenchymal cells, which can induce bone formation. Grafts that are osteoconductive provide a matrix to support the ingrown bone, which requires the presence of the host for bone healing (Roberts et al., 2012). The synthetic bone grafts resorption creates porosity while it stimulates bone growth, as it can help in the shaping of the paste to the complex bone cavity defect. The cements are also injectable, ideal for minimally invasive procedures (Fernandez de Grado et al., 2018).
Calcium phosphate (Ca3(PO4)2) is one of the commonly used synthetic bone graft substitutes (Bohner et al., 2020). It has been commonly used in the areas of dentistry for tooth replacements, but recent research has pointed to its use as a bone graft in malignant bone tumors (Al-Sanabani et al., 2013; Friesenbichler et al., 2017). Research has shown that calcium phosphate has the material property of ceramics with bioabsorbable additives that may improve mechanical strength, which can lead to cell growth in the cement. However, the disadvantages of calcium phosphate bone grafts include its brittle nature, reduced rates of resorption, and poor performance at higher temperatures. This can lead to a decrease in overall mechanical strength and differences in degradation rate as well as the relationship between ingrowth and pore size (Eliaz and Metoki, 2017). These limitations of calcium phosphate require bio-friendly polymers, which can increase the overall mechanical strength and provide a faster rate of reabsorption of the graft.
Engineered Cement Composites Model
Thus, this project utilizes the research conducted by Dr. Victor Li and his team, which focuses on a concrete model called Engineered Cementitious Composites (ECCs) (Li, 2019). The study mainly focuses on methodology of strain-hardening cement composites. Over the last several decades, concrete with increasingly high compressive strength has been used for structural applications. Incorporating micromechanics and this materials design theory allows for microstructure tailoring and materials optimization, enabling flexible materials processing. Most of these materials, however, remain brittle, with low ductility. In certain locations, where steel and concrete come into contact or in connections of steel and concrete hybrid structures, the high stress concentration created can lead to fracture failure of the concrete where the matrix cracking strength (including the first crack strength) exceeds the maximum bridging stress. These unique properties of the ECC model such as enhanced crack width and tensile ductility initially inspired this project to synthesize synthetic bone grafts and improve their low mechanical properties through using hybrid structures, such as fiber reinforced concrete (Li, 2019).
This has spurred an investigation into developing a more cost-effective, highly ductile cementitious material suitable for structural applications. The advantages of the composite in the hardened state and flexible processing in the fresh state make the ECC attractive for a broad range of applications. The microcracks formed in the composite surrounding fiber reinforcement are essential for high bendability and ductility, as it allows the load stress to distribute more evenly on the surface area (Li, 2019). In terms of material constituents, the ECC does not use large amounts of fiber to optimize the composite strength. In general, ≤ 2% by volume of discontinuous fiber in high-aspect ratio is adequate for strain-hardening behavior. However, the ECC has also shown improvements in ductility, as it is primarily controlled through disordered motion where the polymer fibers can stretch and move easily during stress. Thus, toughness is a great indicator of ductility, as it relates to bond strength and mobility of molecules in a material. At higher fiber volumes (> 2%), large cracks can be induced due to bridging action of aggregates, cement ligaments, and fibers (Li, 2019). At these volumes, the ductility of the material becomes too high and reaches a breaking point, which adversely affects the fiber bridging properties and decreases the mechanical strength. Thus, at low fiber volumes, these materials have excellent mechanical properties, potentially including improvements in hardness and toughness (Li, 2019). This material design should not only result in higher strain-hardening to localized plastic deformation but also higher toughness when a high load is applied.
By incorporating the ECC design, we aim to reinforce the calcium phosphate synthetic bone matrix upon the addition of gelatin and cellulose polymers, which are otherwise too brittle when in the hardened state. Gelatin was selected due to its robust biocompatibility and biomimicry that it shares with collagen in the extracellular matrix (Kim et al., 2005). The presence of gelatin has been found to provide a favorable environment for osteoblast-like cells, resulting in cell proliferation and mineralized tissue formation. Silicate and gelatin-based materials have also been seen to foster osteoblast adhesion and growth and have been used as implant material for bone repair (Ding et al., 2011). The presence of gelatin can improve the mechanical properties of the ceramic cement due to a more even distribution of the mechanical load via interactions with cellulose fibers as well as lower porosity (Ding et al., 2011).
Cellulose-Gelatin Chemistry and Bonding
Cellulose nanocrystals were chosen as structural reinforcement because of their exceptional mechanical properties and biocompatibility in their fibrous form when dissolved. Cellulose is a diverse material with tunable properties and is a promising platform for biomaterial development (Hickey and Pelling, 2019). These natural polymers exhibit a unique scaffolding property that is able to support cell adhesion, migration, growth, and direct stem cell differentiation. This can be influenced by the scaffold'’s chemical composition, stiffness, surface topography, geometry, and permeability (Hickey and Pelling, 2019).
Regarding its chemical properties, cellulose is a compact sugar made up of monosaccharide glucose molecules. The polymer consists of repeated glucose units that are attached together by β-1,4 glycosidic linkages. The β-1,4 glycosidic bond is formed by covalent bonding of oxygen to the C1 of one glucose ring and the C4 of the connecting ring (Liu et al., 2022). The hydroxy groups in the repeating units’ ability to hydrogen bond between cellulose chains are responsible for cellulose’s physical properties.
Research has shown that hydrogen bonding between cellulose and gelatin may play a predominant role in improving the structural integrity and mechanical properties of gelatin film (Liu et al., 2022). Considering the chemical structure of gelatin and cellulose, the carbonyl and amide groups of gelatin in the peptide linkage are capable of forming intermolecular hydrogen bonds with the hydroxy groups in cellulose. These intermolecular interactions can aid in transferring the load stress between cellulose and gelatin (Liu et al., 2022). At low fiber volumes, the cellulose-gelatin crosslinking can enhance the bone graft’s mechanical properties. Based on the ECC model, we hypothesize that the bone graft reinforcement of synthetic CNC will increase the hardness and toughness of the bone matrix up to a critical volume ≤ 2% followed by a gradual decrease due to fiber agglomeration, which induces high fracture failure and decreases hydrogen bonding between cellulose and gelatin (Li, 2019).
Project Aim and Objectives
The ultimate goal of this project is to mechanically optimize commercial injectable bone grafts and enhance their use in diverse and higher load-bearing clinical applications. Therefore, bone grafts with sound mechanical strength will be produced. To achieve this goal, we plan to investigate several objectives. The first objective is to provide evidence of bone graft mechanical enhancement in hardness and toughness up to a critical value as a function of cellulose concentration (v/v %). In this study, we anticipate that both graphs of average hardness and toughness with respect to cellulose concentration will have a moderate to strong Pearson’s product-moment correlation coefficient (Pearson’s r). Our evaluation of a success metric considering the ECC model is a linear and correlated increase in the defined mechanical properties up to the critical volume ≤ 2% cellulose followed by a decrease due to rapid fiber aggregation, which causes cracking and fracture failure along with decreased fiber hydrogen bonding. Multiple bone graft replicates from a few batches will be made to demonstrate consistency and corroborate the proposed hypothesis.
Another objective of this study is to demonstrate and confirm the chemical composition of synthetic bone grafts, specifically the presence of cellulose and gelatin fibers. In the pure-reagent FTIR graphs, we anticipate the presence of calcium phosphate ionic bands in the fingerprint region along with peaks representing carbonyls, amides, and hydroxy groups in cellulose and gelatin. However, in the overlaid FTIR spectra of our bone graft samples, the hydroxy and amide peaks are expected to have a decreased intensity and frequency, which confirms the crosslinking and bonding state of cellulose and gelatin and verifies the samples to be applicable for mechanical testing.
The next objective is to characterize the degree of hydrogen bonding between cellulose and gelatin polymers as a function of cellulose concentration (v/v %). Not only can FTIR show the chemical composition of our samples, but research has shown that it can also provide evidence of the degree of hydrogen bonding between cellulose and gelatin within the fingerprint region around a wavenumber range of 1000–1100 cm-1 (Lu et al., 2018). These peaks are represented in terms of their broadness, where the broader the peak is, the greater the cellulose-gelatin hydrogen bonding. Thus, we expect to see a correlation of the broadness in these peaks with respect to the mechanical strength, as more cellulose fibers will hydrogen bond with the gelatin polymers before the 2% critical point, after which there will be less hydrogen bonding due to fiber agglomeration within the calcium phosphate matrix.
The final objective of this project is to provide visual evidence via microscopy of cracking planes and cellulose-gelatin interactions within the bone grafts. This will help better depict the surface morphology and cellulose-gelatin bonding distributions. This should corroborate the mechanical and FTIR data in that low-fiber CNC reinforcement can enhance mechanical properties due to scattered microcracking and ordered crosslinking while high-fiber CNC reinforcement leads to catastrophic failure and fiber aggregation.
Materials and Methods
Bone Graft Synthesis
Bone cement composites with CNC concentrations of 0 to 5% will be synthesized for sufficient comparative analysis of the relationship between low fiber volumes and hardness, toughness, and intermolecular hydrogen bonding. To start, a 10 mL mixture of organic solvents tetrahydrofuran (THF; Fisher Chemical, U.S.A.) and N,N-dimethylformamide (DMF; Fisher Chemical, U.S.A.), with ratio 98:2, respectively, was prepared to dissolve the cellulose. This ensured that the cellulose was uniformly dispersed into soluble fibers before it was mixed together in the formation of the bone graft. This mixture was agitated preliminarily for five minutes via ultrasonic cavitation to promote uniformity.
The mass of cellulose nanocrystals (8.0 w/w% aqueous gel; Blue Goose Biorefineries, Inc.TM; Canada) was then weighed according to the percent composition being tested, beginning with a control without cellulose and increasing to percentages 1–5% cellulose by volume. The cellulose was then added to the solvent mixture, and the mixture was agitated via ultrasonic cavitation for 30 minutes and then magnetically stirred 30 minutes, after which the mixture was homogeneous.
For the base of the graft, 10 g of calcium phosphate dibasic dihydrate (CaHPO4•2H2O; 98+%, extra pure; Fisher Chemical, U.S.A.) were weighed out. 0.2 g of type A (porcine) gelatin (MP Biomedicals, LLC, U.S.A.) were then dissolved in 7.15 mL distilled water according to the approximate ratio of gelatin to water (0.75 oz gelatin per cup of water), so that the gelatin would be dissolved before being vacuum mixed (Alters, 2019). This solution of gelatin and water was magnetically stirred for 30 minutes before vacuum mixing.
Sodium metasilicate (Na2SiO3; technical grade; Fisher Chemical, U.S.A.) was used to promote the solid formation between the substrate calcium phosphate and the gelatin-cellulose reinforcing network (Lakrat et al., 2022). 3.85 mL of silicate were measured to obtain a 0.4 liquid-to-powder ratio of sodium silicate to calcium phosphate, and this volume was stored until vacuum mixing (Rabiee and Baseri, 2012).
Vacuum Mixing
All reagents were combined into a vacuum mixer, and the contents were hand mixed for 2 minutes. Upon mixing, a white paste was observed.
Upon thorough vacuum mixing and solidification, the product was added into 5 mm dyes. The sample was allowed to dry in a vacuum oven set at 40 KPa and 23°C for one week. Indicating Drierite® (8 mesh; mixture of CaSO4 and CoCl2• 6H2O; Thermo Fisher Scientific, Inc., U.S.A.) was set beside the mixture to remove any excess water and to solidify the product so that it could be prepared for mechanical testing.
Hydraulic Pressing
Once dried, the sample was then crushed with a mortar and pestle and pressed into thin pellets via hydraulic press (specimen mount press, Buehler™, U.S.A.). To account for the dyes’ thickness, 0.5 g were used for the hydraulic press, and each sample was pressed under a pressure of approximately 41 MPa.
Vickers Testing
A total of 72 pellets were synthesized for analysis using the Micro Vickers Hardness Tester (Phase II+™, U.S.A.) under a magnification of 10x and load of 9.8 N. The Vickers hardness of each pellet was calculated by averaging the hardness measured at 5 sites per pellet: middle, right, bottom, top, and left. The hardness at each site was determined by measuring the distances of the 2 diagonals in the rhombic indentation (in μm) via the four corners function in the Micro Vickers Hardness Tester software, iVision v.1.0.0 (iVision Software, Canada). In order to provide an accurate representation of the data collected, the reported hardness values are the means of the 12 indented pellets for each cellulose concentration.
Toughness
Separate bone-graft pellets (total = 90 pellets) were made via hydraulic pressing for toughness testing using the MTS 810 (MTS®, U.S.A.). The data was subsequently analyzed using MTS Flex SE (MTS®, U.S.A.). Each pellet was placed on the lower compression platen with a compression rate of 1 mm/min. The load was started when the opposite compression platen contacted the sample and continued until the point of fracture (measured with the first decrease in the maximum force value), and stress-strain curves were generated to calculate toughness. The toughness is represented by the area under the curve from the first positive strain value to the strain value of maximum stress, or specific energy (N.m/m3, or J/m3). The reported toughness values are the means of 15 indented pellets for each cellulose concentration.
FTIR Spectroscopy
Bone graft samples with CNC concentrations of 0 to 5%, along with pure samples of calcium phosphate, cellulose, and gelatin were compared with Fourier-transform infrared (FTIR) spectroscopy. The pellets were scanned with a Bruker Alpha FTIR spectrometer (Bruker, U.S.A.) set to 16 scans in the range of 4000–400 cm-1 at a resolution of 4 cm-1, and OPUS 7.2.139.1294 (Bruker, U.S.A.) was used to display and analyze the spectra. Testing was conducted for the pure reagents used (calcium phosphate, cellulose, and gelatin) along with the bone graft samples in order to compare their functional groups. Note that the spectra representing the 1–5% cellulose concentrations were superimposed for clearer comparison between the samples’ chemical compositions and the pure polymers. Such superimposition was possible because there were small differences in transmittance at this scale. Further analysis was needed to examine the degree of hydrogen bonding as a function of cellulose concentration, where the broadening of transmittance values were calculated between the local maximum peaks in the 1000–1100 cm-1 wavenumber range (Lu et al., 2018).
SEM
Pellets of all cellulose concentrations (0-5 v/v %) that were subjected to toughness testing were sputter coated with Au-Pd for several minutes using the Denton Vacuum Desk V (Denton Vacuum, U.S.A.). The pellets were then imaged at various magnifications ranging from 500x-50,000x using the FEI Quanta 650 FEG SEM (Thermo Fisher Scientific, Inc. U.S.A.).
Statistical Analysis
SPSS® v. 29.0.0.0 (IBM®, U.S.A.) was used to conduct a one-way ANOVA and Tukey post-hoc test to evaluate statistical significance in the hardness and toughness values across and between cellulose concentrations. Pearson’s r was also calculated to evaluate the linear correlation between cellulose concentration and mean hardness and toughness. Statistical significance was reported as p < 0.05.
Results
Vickers hardness is not significantly enhanced at low CNC volumes but decreased past the critical volume fraction
The results display the plot of mean Vickers hardness versus percent cellulose by volume, with the error bars representing standard error (Figure 1). A linear increase in hardness was observed until a critical volume of 2 v/v% CNC (r = 0.5761), after which a sharp linear decrease (r = -0.9986) was seen. Based on the one-way ANOVA, high statistical significance between mean hardness values was observed across groups (p < 0.001), allowing the rejection of the null hypothesis of equal hardness means across cellulose concentrations. Post-hoc testing showed significance specifically between the following cellulose concentrations: 2% and 3%, 2% and 4%, and 2% and 5%. There was no statistical significance between concentrations 0% and 1%, 0% and 2%, and 1% and 2%. Thus, the Vickers hardness of the bone graft samples was not statistically different with respect to the cellulose fiber volume up to a critical volume of 1–2%, but subsequent significant drops in hardness followed after exceeding the critical point.
Figure 1. Plot of Average Vickers Hardness (MPa) vs Cellulose Concentration (v/v%) with error bars representing Standard Error. The Vickers hardness of each pellet (total = 72 pellets, 12 per cellulose concentration) was calculated by averaging the hardness measured at 5 sites per pellet using the Micro Vickers Hardness Tester (Phase II+™, U.S.A.) under a magnification of 10x and load of 9.8 N. The hardness at each site was determined by measuring the distances of the 2 diagonals in the rhombic indentation via the four-corners function in the Micro Vickers Hardness Tester software, iVision v.1.0.0 (iVision Software, Canada). The average Vickers hardness did not statistically change with cellulose concentration until after 2% v/v with subsequent decreases in hardness.
Toughness is not statistically different between any two CNC concentrations
The results display the plot of mean fracture toughness versus percent cellulose by volume, with the error bars representing standard error (Figure 2). The one-way ANOVA showed statistical significance across fracture toughness values (p = 0.038), so the null hypothesis of equal mean toughness values across cellulose concentrations was rejected. Post-hoc testing showed no significance between any two cellulose concentrations. The disparity in the significance results of the ANOVA and post-hoc tests can be explained by the lack of statistical power in this experiment given the groups had relatively small sample sizes (15 per group). Since the post-hoc test is less statistically powerful in terms of its comparison groups unlike multiple comparison testing in ANOVA, it is less likely to detect significance.
Figure 2. Plot of Average Fracture Toughness (MPa) vs Cellulose Concentration (v/v%) with error bars representing Standard Error. The fracture toughness of each pellet (total = 90 pellets, 15 per cellulose concentration) was calculated using the MTS 810 (MTS®, U.S.A.) and analyzed with the MTS Flex SE (MTS®, U.S.A.). The load was started when the opposite compression platen contacted the sample and continued until the point of fracture, and stress-strain curves were generated to calculate fracture toughness represented by the area under the curve from the first positive strain value to the strain value of maximum stress. There was no statistically significant difference in fracture roughness between any two cellulose concentrations.
FTIR spectroscopy confirms cellulose and gelatin increased hydrogen bonding with low CNC reinforcement and decreased hydrogen bonding after critical volume fraction
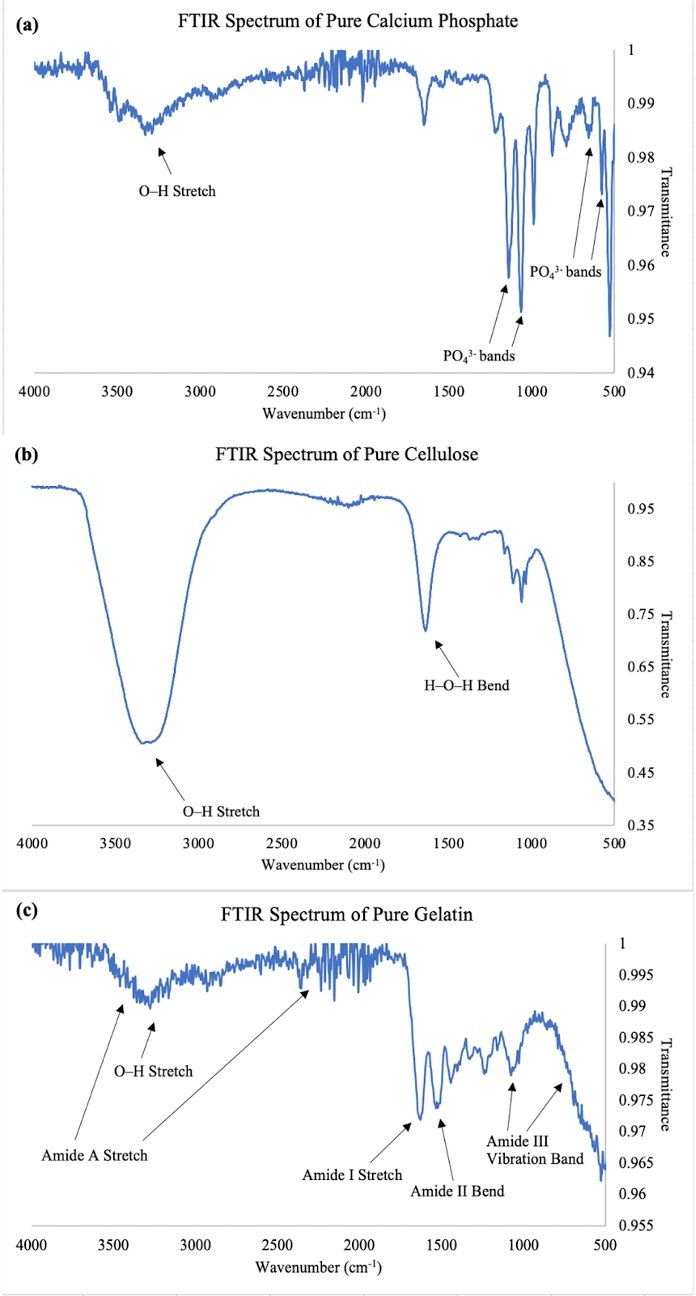
The spectra of the pure reagents are displayed, with peaks of interest labeled (Figure 3). The presence of PO43- ionic bands at 547, 657, 1063, and 1139 cm-1 in the calcium phosphate spectra denotes the four vibrational modes of PO43- , and an O-H stretch is also depicted from 3200–3500 cm-1 (Figure 3a; Berzina-Cimdina and Borodajenko, 2012). An O–H stretch is also seen in the pure cellulose spectra from 3200–3400 cm-1 along with an H-O-H bend at 1636 cm-1 (Figure 3b).
The four amide bands characteristic of porcine gelatin are observed in the pure gelatin spectrum (Figure 3c; Pradini et al., 2018). The amide A stretch at 3461 cm-1 corresponds to N–H stretching vibrations, representing the amide groups present, and merges with the O–H stretch from 3200–3400 cm-1, which represents the free hydroxy group in the hydroxyproline residue in the protein monomer (Pradini et al., 2018). The amide I band at 1628 cm-1 reflects the C=O stretching in the carbonyls throughout the polypeptide, while the amide II signal at 1521 cm-1 shows N-H bending and C-N stretching. Finally, the amide III bands at 1075 and 714 cm-1 signal C-N bond-plane vibration, confirming the gelatin’s presence (Pradini et al., 2018).
Figure 3. FTIR Spectra of Pure Reagents Used. (a) Calcium Phosphate. (b) Cellulose. (c) Gelatin. All pellets were scanned with a Bruker Alpha FTIR spectrometer (Bruker, U.S.A.) set to 16 scans in the range of 4000–400 cm-1 at a resolution of 4 cm-1, and OPUS 7.2.139.1294 (Bruker, U.S.A.) was used to display and analyze the spectra. The wavenumber is represented on the x-axis while the transmittance is represented on the y axis. The characteristic peaks of each pure reagent are labeled and confirm the respective functional groups.
Evaluating the spectra of the bone cements relative to the pure samples (Figure 4), the O-H stretch from pure cellulose is largely diminished as well as redshifted to 3020-3050 cm-1. Additionally, the amide I and II bands in pure gelatin are redshifted in the 1-5% to 1583 cm-1 and 1493 cm-1 respectively. The final results show a comparison of broadening in transmittance shifting in the 1000-1100 cm-1 range, which was used to evaluate degrees of hydrogen bonding between samples as a function of cellulose concentration (Figure 5; Lu et al., 2018). The control exhibits a weak transmittance shift, followed by the broadest shifts of 1% and 2% cellulose. The 3% and 4% display narrower transmittance shifts, aligning with the trend observed in the Vickers hardness results (Figure 1). Note that the 5% shift is slightly broader than the critical 1 v/v%, which could be due to undissolved cellulose.
Figure 4. FTIR Spectral Comparison of Pure Polymers to Bone Graft Samples. Similar methods were conducted as discussed in Fig. 3. Note that the pure polymers and samples are color coded and overlayed as shown by the legend. The hydroxyl stretches and amide bands in cellulose and gelatin respectively are found to be shifted to higher wavelengths compared to Fig. 3 (3020–3050 and 1583-1493 cm-1 respectively).
Figure 5. Comparison of FTIR Transmittance Broadening in Bone Graft Samples. Testing was similar as mentioned in Fig. 3 and 4, but further analysis was needed to examine the degree of hydrogen bonding as a function of cellulose concentration, where the broadening of transmittance values was calculated between the local maximum peaks in the 1000–1100 cm-1 wavenumber range. The transmittance values are represented on the x axis while the cellulose concentrations are represented on the y axis. The 0% cellulose sample displays the weakest transmittance followed by the broadest shifts of 1% and 2% cellulose. The 3% and 4% display narrower transmittance shifts, aligning with the trend observed in Figure 1. The 5% shift is slightly broader than the critical 1 v/v%, which could be explained by undissolved cellulose.
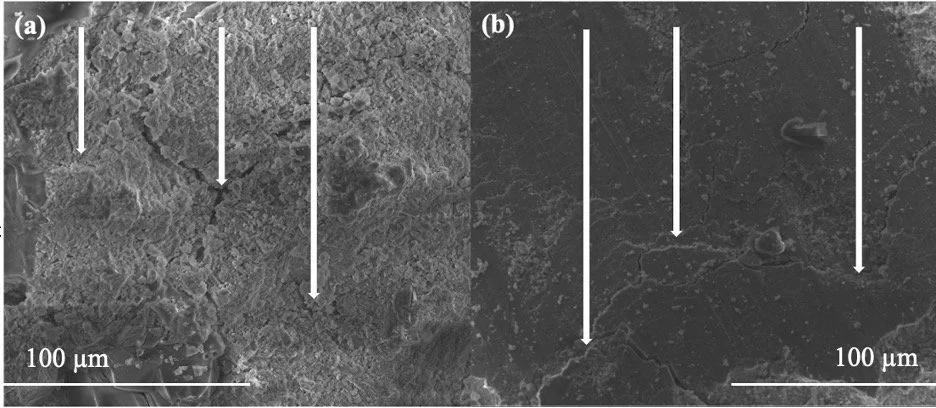
SEM displays large cracking and fiber aggregates in high-fiber cement compared to microcracks and ordered crosslinking in low-fiber cement
Images of low-fiber (≤ 2 v/v%) and high-fiber (> 2 v/v%) CNC samples, respectively, are displayed at a magnification of 1600x (Figure 6). Examining degrees of microcracking within the low-fiber cements, microcracks are scattered throughout the composite and appear to propagate in distinct regions (Figure 6a). However, in the high-fiber cement, the cracks appear to be substantial, spanning across the entire surface (Figure 6b).
Figure 6. SEM Images of Composite Cracking. (a) Low-Fiber Cement. (b) High-Fiber Cement. Pellets of all cellulose concentrations that were subjected to toughness testing were sputter coated with Au-Pd for several minutes using the Denton Vacuum Desk V (Denton Vacuum, U.S.A.). The pellets were then imaged at a magnification of 1600x to evaluate composite cracking. The arrows are presented in each image to delineate areas of cracking. In 6a, microcracks appear scattered throughout the composite and propagate in distinct regions. In 6b, the cracks appear to be more substantial and span across the entire surface.
The high-fiber volume CNC samples are displayed at a magnification of 12000x (Figure 7). Ordered Cellulose-gelatin crosslinking looks to be present in the left-hand image, evidenced by the heterotypic fiber interactions (Figure 7a). A large, disordered conglomerate of fibers is also observed in the right-hand image, however, with most of the fibers appearing homotypic (Figure 7b).
Figure 7. SEM Images of High-Fiber Composite. (a) C-G Crosslinking. (b) Aggregation. Similar testing was conducted to evaluate fiber crosslinking as described in Fig. 6 except at a magnification of 12000x. In 7a, the crosslinking appears to be ordered by the heterotopic fibers. In 7b, a large aggregate of fibers is observed with most of them appearing homotypic.
Discussion
Vickers Hardness
The hardness trend that is ideally characterized by a gradual increase in strain-hardening behavior up to a critical volume fraction can be explained by the micromechanics approach of the ECC model, where generally low fiber volumes (≤ 2%) can improve the frictional bonding of the matrix without causing fiber breakage (Li, 2019). This can be further rationalized by the properties of cellulose and gelatin, where strong hydrogen-bonding interactions between the hydroxy, carbonyl, and amide groups can improve the frictional bonding of the matrix, which can aid in spreading out the localized load stress (Liu et al., 2022). In theory, this more even distribution should lead to greater strain-hardening behavior, where the low-CNC-volume composites should display higher resistance to localized plastic deformation from the indenter. However, the results indicate that there is no significant difference in Vickers hardness between the control sample and 1 and 2% CNC reinforcement (Figure 1). These results can imply that cellulose reinforcement is not conducive to higher tolerance for surface-level tolerance below the critical volume fraction despite a higher degree of hydrogen bonding between cellulose and gelatin, as seen in the FTIR analysis.
At CNC volumes > 2%, the hardness drastically decreases, with significant differences observed between subsequent samples. As mentioned previously, research has shown that at higher fiber volumes, fracture failure can occur due to the aggregation of fibers, which prompts decreased hydrogen bonding, as observed in the FTIR analysis (Li, 2019). This supports the fact that the surface-level load resistance of the bone graft composite lowers at higher fiber volumes due to the fibers’ agglomeration. It is likely that this agglomeration prompts higher internal cracking inside the composite and decreases the surface hardness of the composite, as it is more prone to higher penetration from the indenter (Li, 2019). Overall, this analysis implies that increasing amounts of CNC reinforcement after the critical volume fraction are inversely correlated with strain-hardening behavior of the bone graft sample.
Toughness
The lack of significant differences in toughness with CNC reinforcement are likely attributed to several reasons (Figure 2). One possibility is that the relative sample size was small compared to other tests conducted in this study. The lack of a large sample size could have prevented the data from being extrapolated into a clear mechanical trend, leading to indeterminate conclusions. Another potential reason is that the polymer fibers may not have been adequately dissolved and mixed during the synthesis process since it was observed that cellulose and gelatin had a tendency to clump together in small pieces even after they were mixed in their respective solvents. While this error could have been prevented by longer durations of mixing at increased speeds to break all clumps, the lack of complete homogeneity could imply that the fibers were not uniformly distributed within the bone graft. Given that the MTS compresses the entire bone graft pellet and induces cracks as opposed to the Micro Vickers Hardness Indenter, which mainly assesses surface-level behavior, inadequate fiber distribution may have led to early compressive failure via large cracking within the matrix. This is because the lack of consistent fiber bridging in the sample may have reduced the ductility of the bone graft pellet through reduced composite microcracking and polymer stretching across the sample and instead promoted rapid fracture failure (Li, 2019).
The significant disparity between the 0% and the 5% CNC sample can be explained by the fact that the 5% sample likely contained undissolved cellulose. It could be that unreacted cellulose played a role in increased intramolecular hydrogen bonding (Figure 5), which could result in higher toughness due to increased matrix friction and ductility (Lu et al., 2018). Overall, the results are inconclusive on whether CNC reinforcement can enhance toughness properties of calcium phosphate bone graft samples.
However, rejection of the null hypothesis implies that CNC reinforcement has the potential to increase the toughness of calcium phosphate bone cement. With a larger sample size, thorough fiber synthesis, and minimization of other confounding variables, CNC reinforcement may increase the higher toughness of bone graft samples up to a critical CNC volume of 1–2% followed by a decrease due to fiber aggregation and the lack of fiber bridging properties amid an applied force similar to the hardness results (Figure 1). In theory, CNC uniformity should prompt composite microcracks within the calcium phosphate matrix and allow the load stress to distribute to other deep areas within the bone graft pellet, reflecting the increased toughness values (Li, 2019). This could potentially reveal that toughness is correlated with hardness at the nanoscale level up to a critical fiber volume, which could have clinical implications for creating an enhanced mechanical bone cement composite with higher strain capacity and ductility.
FTIR Spectroscopy
The PO43- peaks in the calcium phosphate spectra (Figure 3a) also confirm the bonding between PO43- and Ca2+ in the compound tested (CaHPO4•2H2O; Berzina-Cimdina and Borodajenko, 2012). Furthermore, the O–H stretch confirms the hydration of the ionic compound (Berzina-Cimdina and Borodajenko, 2012).
The O–H stretch in the pure cellulose spectrum (Figure 3b) coincides with cellulose’s structure, as hydroxy groups protrude out of the plane from the polysaccharide structure while the H–O–H bend signifies the presence of the aqueous medium in which the cellulose was dissolved (Hospodarova et al., 2018). As seen in subsequent results (Figure 4), such redshifting indicates intermolecular hydrogen bonding between the hydroxy and amino groups in the polymers, which confirms cellulose and gelatin fiber bonding within the samples (Lu et al., 2018).
Turning to the comparison of the broadening of transmittance values among the bone cements (Figure 5), the broader transmittance shift from 0% to 1% and 2% and then gradual reduction past suggests that ~ 1 v/v% is a critical CNC volume. This sloping coincides with the cleavage of cellulose’s β-1,4 glycosidic bonds linking its glucose monomers upon dissolution, meaning that 1% appears to reflect maximal cellulose dissolution, which supports the peak hardness (Figure 1; Lu et al., 2018). Importantly, when cellulose dissolves, its inter- and intramolecular hydrogen bonds break, leading to higher hydroxy-group interaction (Lu et al., 2018). Dissolved CNC, therefore, can readily crosslink with other polymers (such as gelatin) via hydrogen-bond reformation, which is implied by the spectra (Lu et al., 2018).
The reduced transmittance shifting past 1% CNC, which reflects an altered dipole that could be caused by diminished hydrogen bonding, is thought to be due to fiber aggregation. There appears to be an optimal amount of hydrogen bonding around 1–2 v/v%, after which CNC and gelatin are believed to clump intramolecularly so that crosslinking is reduced. This also seems to support the reduction of hardness after the critical 1–2% CNC volume due to reduced crosslinking (Figure 1). With less cellulose-gelatin interaction, the cement is afforded less support, leading to higher rates of catastrophic failure under higher loads due to the diminished ability to adequately distribute the load across the matrix (Li, 2019).
Regarding the 5% peak, it could be that precipitated, unreacted cellulose is the primary cement component in the registering vibrations. In surpassing cellulose’s solubility limit, unreacted cellulose would be expected to yield stronger absorbances and broader transmittance shifts due to the high polarity of the free hydroxy groups (Lu et al., 2018).
SEM
The formation of composite microcracks in the low-fiber cement (Figure 6a) appear to form when subjected to high loads, which allows the load stress to distribute more evenly across the surface area due to the extensive stretching of the polymer fibers. Based on the ECC model, these properties are conducive to higher strain-hardening behavior and ductility (Li, 2019).
However, in the high-fiber cement (Figure 6b), substantial cracks could indicate catastrophic failure, where larger cracking can be induced due to lower fiber-bridging support across the matrix. When the critical fiber volume is exceeded, the material’s ductility becomes too high and reaches a breaking point. Regarding the fiber interactions in the high-fiber cement (Figure 7), the apparent aggregation confirms the reasoning behind the Li model, where polymers in ECCs with high-fiber volumes are presumed to self-assemble more than crosslink between each other (Li, 2019). Moreover, the agglomeration corroborates the Vickers hardness and FTIR results, where self-assembly past the critical CNC concentration led to significantly diminished hardness as well as lower transmittance shifting from reduced hydrogen bonding.
Improvements and Future Outlook
By mimicking the ECC model, this study attempted to mechanically optimize calcium phosphate bone grafts with CNC reinforcement along with gelatin fibers, which incorporates a high-strength calcium phosphate matrix. While the results did not display statistically significant increases in hardness or toughness with CNC reinforcement, obvious decreases in hardness were seen after the 1–2% critical value. This decrease was further supported by the FTIR analysis, which not only displayed evidence of cellulose and gelatin hydrogen bonding through redshifting but also overall decreases in transmittance broadening after the 1–2% CNC critical point, indicating a decrease in cellulose and gelatin bonding. Thus, it is plausible to think that decreases in hardness properties are due to higher internal cracking inside the composite due to less fiber-bridging properties amid the force applied on the bone graft. SEM also showed that low- fiber CNC samples exhibited extensive microcracking compared to higher-fiber CNC samples, which contained larger cracks and fiber aggregates. Overall, this analysis implies that high-fiber CNC reinforcement is antagonistic to strain-hardening behaviors and potentially ductility due to decreased cellulose-gelatin hydrogen bonding.
In terms of limitations of this study, the lack of significant changes in toughness observed with CNC reinforcement can be attributed to the small sample sizes as well as the possibility that the polymer fibers were not adequately uniform within the bone graft due to incomplete dissolution. These limitations could impact the statistical significance in the results and lead to incomplete extrapolation of clear mechanical trends. However, a better fiber synthesis technique could yield more significant results due to better fiber distribution and bridging properties across the matrix cracks when subjected to high loads. These techniques could include electrospinning in order to better control the fiber distribution within the matrix and also UV crosslinking to promote polymer bonding. Regardless, with a greater sample size and improved techniques, this study has potential to display enhanced strain-hardening behavior and ductility in commercial bone grafts with low-fiber CNC reinforcement.
The main future goal of this project is to confirm the effectiveness of the bone graft reinforced with low-fiber CNC through clear evidence of enhanced mechanical properties, such as hardness and toughness. Another goal of this project is to display the biocompatibility of the bone graft samples for clinical applications. Clinical parameters such as injectability and porosity are pertinent to this project and can be investigated (Eliaz and Metoki, 2017). In the context of this project, we anticipate that CNC reinforcement is conducive to adequate pore size and injectability, which will help confirm the bone graft’s clinical reliability.
Acknowledgements
A special thanks goes to my mentor, Aaron Catledge, who gave me guidance and helped along the way so that the project could continue in a clear direction. I would also like to thank my lab assistants, John Medley and Sean Phan, who helped me in experimentation, data analysis, and manuscript writing. Finally, I would like to recognize Haibin Ning and Yan Yongzhe for contributing to the toughness data collection and analysis, along with Kirsten Pittman for helping with SEM and computing.
Conflicts of Interest/Disclosure
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
Al-Sanabani, J. S., Madfa, A. A. and Al-Sanabani, F. A. (2013) ‘Application of Calcium Phosphate Materials in Dentistry’, International Journal of Biomaterials, 1-12, available: www.doi.org/10.1155/2013/876132.
Alters, M. (2019) Determination of Clinical Efficacy of Ultrasound Stimulation on Piezoelectric Composites for Power Generation Applications, unpublished thesis (M.S.), Kansas University, available: https://kuscholarworks.ku.edu/handle/1808/30493.
Berzina-Cimdina, L. and Borodajenko, N. (2012) ‘Research of Calcium Phosphates Using Fourier Transform Infrared Spectroscopy’ in Theophanides, T., ed., Infrared Spectroscopy - Materials Science, Engineering and Technology, Latvia: InTechOpen, available: http://dx.doi.org/10.5772/36942.
Bohner, M., Santoni, B. L. G. and Döbelin, N. (2020) ‘β-tricalcium phosphate for bone substitution: Synthesis and properties’, Acta Biomaterialia, 113, 23-41, available: www.doi.org/10.1016/j.actbio.2020.06.022.
Dimitriou, R., Jones, E., McGonagle, D., and Giannoudis, P. V. (2011) ‘Bone regeneration: current concepts and future directions’, BMC Medicine, 9(66), available: www.doi.org/10.1186/1741-7015-9-66.
Ding, S.-J., Wei, C.-K. and Lai, M.-H. (2011) ‘Bio-inspired calcium silicate– gelatin bone grafts for load-bearing applications’, Journal of Materials Chemistry, 21(34), 12793, available: www.doi.org/10.1039/c1jm11171j.
Eliaz, N. and Metoki N. (2017) ‘Calcium Phosphate Bioceramics: A Review of Their History, Structure, Properties, Coating Technologies and Biomedical Applications’, Materials, 10(4), 334, available: www.doi.org/10.3390/ma10040334.
Fernandez de Grado, G., Keller, L., Idoux-Gillet, Y., Wagner, Q., Musset, A.-M., BenkiraneJessel, N., Bornert, F. and Offner, D. (2018) ‘Bone substitutes: a review of their characteristics, clinical use, and perspectives for large bone defects management’, Journal of Tissue Engineering, 9, available: www.doi.org/10.1177/2041731418776819.
Friesenbichler, J., Maurer-Ertl, W., Bergovec, M., Holzer, L.A., Ogris, K., Leitner, L. and Leithner, A. (2017) ‘Clinical experience with the artificial bone graft substitute Calcibon used following curettage of benign and low-grade malignant bone tumors’, Scientific Reports, 7(1), 1736, available: https://doi.org/10.1038/s41598-017-02048-w.
Giannoudis, P.V., Dinopoulos, H. and Tsiridis, E. (2005) ‘Bone substitutes: An update’, Injury, 36(3), 20-27, available: www.doi.org/10.1016/j.injury.2005.07.029.
Hickey, R.J. and Pelling, A.E. (2019). ‘Cellulose Biomaterials for Tissue Engineering’, Frontiers in Bioengineering and Biotechnology, 7, available: www.doi.org/10.3389/fbioe.2019.00045.
Hospodarova, V., Singovszka, E. and Stevulova, N. (2018) ‘Characterization of Cellulosic Fibers by FTIR Spectroscopy for Their Further Implementation to Building Materials’, American Journal of Analytical Chemistry, 9(6), 303-310, available: www.doi.org/10.4236/ajac.2018.96023.
Kim, H.W., Kim, H. E. and Salih, V. (2005) ‘Stimulation of osteoblast responses to biomimetic nanocomposites of gelatin-hydroxyapatite for tissue engineering scaffolds’, Biomaterials, 26(25), 5221–5230, available: www.doi.org/10.1016/j.biomaterials.2005.01.047.
Kumar, P., Vinitha, B. and Fathima, G. (2013) ‘Bone grafts in dentistry’, Journal of pharmacy & bioallied sciences, 5 (Suppl 1), S125–S127, available: www.doi.org/10.4103/0975-7406.113312.
Lakrat, M., Mejdoubi, E.M., Ozdemir, F. and Santos, C. (2022) ‘Effect of sodium silicate concentration on the physico-chemical properties of dual-setting bone-like apatite cements’, Materials Today Communications, 31, available: www.doi.org/10.1016/j.mtcomm.2022.103421.
Laurencin, C., Khan, Y. and El-Amin, S. F. (2006) ‘Bone graft substitutes’, Expert review of medical devices, 3(1), 49–57, available: www.doi.org/10.1586/17434440.3.1.49.
Li, V.C. (2019) ‘Constitutive Modeling of Engineered Cementitious Composites (ECC)’ in Engineered Cementitious Composites (ECC), Heidelberg: Springer, available: www.doi.org/10.1007/978-3-662-58438-5_5.
Liu, Y., Liu, S., Liu, J., Zheng, X. and Tang, K. (2022) ‘Effect of gelatin type on the structure and properties of microfibrillated cellulose reinforced gelatin edible films’, Journal of Applied Polymer Science, 139(19), 52119, available: www.doi.org/10.1002/app.52119.
Lu, Q., Zhang, S., Xiong, M., Lin, F., Tang, L., Huang, B. and Chen, Y. (2018) ‘One-pot construction of cellulose-gelatin supramolecular hydrogels with high strength and pH-responsive properties’, Carbohydrate Polymers, 196, 225-232, available: www.doi.org/10.1016/j.carbpol.2018.05.020.
Office of the Surgeon General (US). (2004) Bone Health and Osteoporosis: A Report of the Surgeon General, Rockville, MD: Office of the Surgeon General (US).
Özcan, M., Hotza, D., Fredel, M.C., Cruz, A., Volpato, C.A.M. (2021) ‘Materials and Manufacturing Techniques for Polymeric and Ceramic Scaffolds Used in Implant Dentistry’, Journal of Composites Science, 5(3), 78, available: www.doi.org/10.3390/jcs5030078.
Pradini, D., Juwono, H., Madurani, K.A. and Kurniawan, F. (2018) ‘A preliminary study of identification halal gelatin using Quartz Crystal Microbalance (QCM) sensor’, Malaysian Journal of Fundamental and Applied Sciences, 14(3), 325-330, available: https://doi.org/10.11113/mjfas.v14n3.942.
Rabiee, S.M. and Baseri, H. (2012) ‘Prediction of the Setting Properties of Calcium Phosphate Bone Cement. Computational Intelligence and Neuroscience, 2012, 1-8, available: https://doi.org/10.1155/2012/809235.
Roberts, T.T. and Rosenbaum, A.J. (2012) ‘Bone grafts, bone substitutes and orthobiologics’, Organogenesis, 8(4), 114-124, available: www.doi.org/10.4161/org.23306
Tay, B., Patel, V. V. and Bradford, D. F. (1999) ‘Calcium sulfate- and calcium phosphate-based bone substitutes: Mimicry of the mineral phase of bone’, Orthopedic Clinics of North America, 30(4), 615-623, available: www.doi.org/10.1016/s0030-5898(05)70114-0