Author: Qingyang Yuan
Institution: University of Maryland
Date: August 2005
Abstract
Spinal cord injury inflicts considerable damage on human physical and physiological regulation and cellular integrity. Although drug therapy and functional electrical stimulation are currently used as treatment, neuronal regeneration in the Central Nervous System (CNS) is crucial for re-establishing neural pathways and function. Regeneration researchers strive to create the most permissive environment for neural growth through strategies such as manipulating gene expression, removing inhibitory factors of glial cells, making antibodies against destructive myelin-associated proteins, injecting neurotrophins and other growth factors, grafting Schwann cells and olfactory ensheathing cells, and even introducing pluripotent stem cells. Both in vitro studies and rat models have shown that these strategies promote axonal extension, plasticity, and some restoration of spinal function, with combination therapies involving tissue graft transplantation and neural growth factors exhibiting the best results. Novel studies using monkeys and less invasive forms of cell replacement point towards more practical solutions for neural repair in humans. In addition to the promising regeneration studies and a few cell replacement therapies undergoing preliminary clinical trials, more research should be done with primate models and safety should be regarded with utmost importance in bringing methods of neuronal regeneration to realistic applications.
Introduction
Over 10,000 cases of traumatic spinal cord injury (SCI) occur each year in the US alone with the total number of cases exceeding 200,000 a figure that doubles when non-traumatic SCI is included (Sadowsky et al. 2002). Males are four times more susceptible to SCI than females (Hulsebosch 2002) and most victims are young - between 16 and 30 years of age (Sadowsky et al. 2002). The majority of SCI is caused by motor vehicle and sports-related accidents, gunshot wounds, and congenital spinal cord defects. Substantial technological advances have increased life expectancy after injury from three months, post-World War II to 20 years in 1966 to 30 years or more in present times (Hulsebosch 2002).
Pathophysiology and Current Treatment
Anatomically, the spinal cord can be injured in four general ways: cord maceration, cord laceration, contusion injury (Figure 1), and solid cord injury. Since spinal nerves affect almost every organ system in the body, in addition to severe impediment of motor function and possible muscle paralysis, SCI causes devastating physiological conditions, including sensory loss, chronic pain, loss of bladder and bowel control, decreased breathing capacity, blood imbalance, thromboembolism, gastric ulcers, and urinary tract infection (Sadowsky et al. 2002; Hulsebosch 2002).
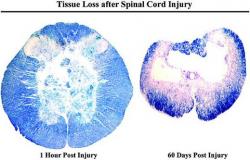
Figure 1. The most popular SCI model is the rodent contusion model, with a necrotic core surrounded by histologically normal-appearing myelinated fibers and portions of grey matter from both dorsal and ventral horns (left). Similar to human SCI pathophysiology, the cell loss continues radially in all directions so that the lesion expands over time. By 60 days post-SCI, there remains only a thin rim of white matter (right). Massive cell death, causing irreversible damage, occurs immediately after the initial impact in the central core region. However, cell death continues to occur over several days and weeks and offers an opportunity for therapeutic intervention to rescue the neural cell populations that are at risk of dying after the first few hours (Hulsebosch 2002).
Histologically, acute SCI damage results in necrosis, severe irregularities in electrochemical potential of the cell, localized edema, demyelination, and tissue hemorrhage. The secondary phase of assault includes release of cytotoxic concentrations of neurotransmitter glutamate, free-radical production, apoptosis, inflammation, slow myelin debris removal, glial production of regeneration inhibitory factors, and the expression of a host of extracellular matrix particles that provide dysfunctional neural guidance (Hulsebosch 2002). Usually astrocytes, the major glial cells, provide the substrate for axonal elongation and trophic factors to support oligodendrocytes. However, after spinal injury, astrocytes proliferate and secrete inhibitory glycoproteins such as chondroitin sulfate proteoglycans (CSPG) (Qiu et al. 2002). When left untreated, the untamed network of astrocytes will form a glial scar, physically barring injured axons from regenerating. Oligodendrocytes, normally axon-protective glial cells, react to injury by producing Nogo, myelin-associated glycoprotein (MAG), and oligodendrocyte-associated glycoprotein (OMgp) that, when bound to the Nogo receptor, repress myelin production and axonal outgrowth (Jones et al. 2003; Li et al. 2004).
Traditional treatment of SCI involves surgery and long-term rehabilitative care such as physical therapy. Nowadays, a number of advanced strategies have proven successful. Methylprednisolone, a glucocorticoid, is a drug effective in suppressing inflammation, but requires a high dosage (Bracken et al. 1992). Tendon transfer involves the transplantation of a tendon from functioning muscle to a non-functioning muscle to restore lost ability. Perhaps the most effective treatment to date is functional electrical stimulation (FES), in which an electrical stimulus applied on the skin, imbedded into the target muscle, or placed around a nerve helps to restore muscle strength and coordination, circulation, respiration, erection and ejaculation, bowel and bladder control, pain control, and ulcer treatment (Sadowsky et al. 2002). Restoring nerve function and integrating neural pathways through neuronal regeneration provides promising options for treatment of SCI.
Neuronal regeneration
Damaged cells of the peripheral nervous system (PNS) are known to regenerate while neurons of the CNS encounter severe difficulty to do so. Unlike the post-injury PNS cellular environment, where myelin and glial scars are cleared and Schwann cells aid re-growth, the injured CNS suffers from a proliferation of inhibitory factors creating an environment hostile for cellular survival (Qiu et al. 2002). Some experiments have demonstrated the intrinsic ability of the CNS to regenerate given the expression of certain proteins like growth-associated protein GAP-43 and the protooncogene bcl-2 (Hulsebosch 2002). However, these reports are not substantive, and major research focuses on inducing a permissive environment for growth with extrinsic factors.
Neuronal regeneration depends on 1) control of glial scars produced by astrocytes, 2) inactivation of myelin-associated proteins, 3) introduction of neurotrophins to enhance growth, 4) replacement of damaged cells by fetal, Schwann cell, or olfactory ensheathing cell (OEC) grafts, and 5) replacement of injured tissue by stem cells (Hulsebosch 2002; Jones et al. 2003). In addition, regenerated fibers need to bridge the gaps caused by gliotic tissue and integrate into the correct neural circuitry to restore proper transmission function. Neuronal plasticity, or flexibility to change morphology, is also important in the guidance of successful regeneration.
Strategies for Neuronal Regeneration
The burst of recent scientific developments and the steady in vivo research in the past few decades uphold strategies in neuronal regeneration as an increasingly more viable treatment option for SCI. However, discoveries are still limited in scope as most animal models are designed to acquire spinal injury by contusion and incomplete lesions - reflecting only a subset of possible human SCI.
Removal of inhibitory factors
Glial fibrillary acidic protein (GFAP) and Vimentin (Vim) are major intermediate filament (IF) proteins of reactive astrocytes that undergo hypertrophy to form the post-SCI glial scar. Menet et al. (2004) found that mice mutant for both GFAP and Vim exhibited reduced gliosis (less nestin immunoreactivity in cells) and increased axonal sprouting of supraspinal neurons responsible for locomotor activity. GFAP-/- and Vim-/- cells bred immature astrocytes, which contributed to an encouraging environment for axonal regeneration. This study showed that while previous experiments involving one knockout (KO) IF gene failed to effectively repress reactive gliosis, a double deficiency in GFAP and Vim is necessary to detect terminal axonal extension and recovery of hindlimb function in rats (Ribotta et al. 2004).
Reactive astrocytes are prone to secrete the proteoglycan CSPG, possessing glycosaminoglycan (GAG) chains that are responsible for its inhibitory activity. To determine the effect of GAG cleavage on spinal cord lesions, Bradbury et al. (2002) delivered the enzyme chondroitinase ABC to the affected dorsal spinal cord regions of adult rats and saw an up-regulation of a neurite growth protein, which promoted regeneration of both ascending sensory and descending motor axons of the corticospinal tract. Some locomotor and proprioceptive function was also restored in the rats.
Another target of intervention is the well-known myelin-associated tri-component protein Nogo, secreted by oligodendrocytes. An antibody, IN-1, has successfully suppressed the cascade of inhibitory effects of Nogo (Merkler et al. 2001) and, along with a Nogo receptor antagonist peptide (GrandPre et al. 2002), has induced the recovery of axonal function in rats with spinal cord injury. Simonen et al. (2003) employed alternative splicing to create Nogo-A KO mice, whose histology exhibited no difference from wildtype mice in immunostaining with MAG, GFAP, CSPG, and other macrophages and microglia; neither were the brain anatomy and myelin ultrastructure different between KO and heterozygous mice. Thus, Nogo-A gene knockout was deemed a treatment agent with mild or little side effects. Regardless, the corticospinal tract fibers of mutant mice clearly extended towards the lesion site more than those of the heterozygous mice. Interestingly, Nogo-A KO mice axons showed similar growth compared to wildtype neurons treated with Nogo-A-specific antibody. Although the expression of Nogo-B and Nogo-C was not tampered, a ten-fold upregulation of Nogo-B in the rat CNS was observed upon its injury. This rise in expression may be due to the inhibitory effects of the Nogo-66 domain, conserved in Nogo-A, -B, and C (Simonen et al. 2003). When Li et al. (2004) intrathecally inserted a soluble, deactivating Nogo-66 receptor in spinal-injured rats, three myelin-inhibiting proteins (Nogo-66, MAG, and Omgp) were blocked, and the density of fibers detected in experimental rats was five times more intense than that of the control. The presence of complex axon sprouting corresponded to an improved neurotransmission and locomotion in affected mice.
The inhibitory effects of myelin-associated proteins can also be reduced by a high quantity of the secondary messenger cAMP. To determine the effect of cAMP on axonal regeneration in vivo, Qiu et al. (2002) injected an analog of cAMP, db-cAMP, directly into the dorsal root ganglion near the L5 lesion site of the rat spinal cord. Compared to the saline control group, all rats injected with db-cAMP showed a greater number of immunolabeled dorsal column axons around the lesion site with regenerated axons ranging from 1.5 mm to 5 mm in length and also greater axonal extension beyond the lesion site with these regenerated axons ranging from 0.6 mm to 0.8 mm in length. This growth almost paralleled the effect of peripheral nerve conditioning, a process in which a cut peripheral nerve transplanted to a dorsal column lesion can instigate CNS axonal regeneration.
Other studies target controlling free radical production, apoptosis, and inflammation as a means to create a permissive environment for regeneration. In one study, autologous macrophages incubated ex vivo with skin cells were injected into rats with spinal contusion 8 to 9 days after injury. High expression of cell surface antigens and MHCII show the macrophages acquiring the characteristics of antigen presenting cells, which effectively hunt local antigens without eliciting cytokine proliferation. The secretion of brain derived neurotrophic factor (BDNF), supportive of axonal growth, and down-regulation of TNFα, infammatory agent inhibitory growth, around the lesion created an environment more supportive of neural development (Bomstein et al. 2003). This study suggests that macrophage activation and uncontrolled inflammation are not synonymous, and that skin-coincubated macrophages may provide relevant features for spinal nerve recovery.
Neurotrophins and other growth factors
In addition to removing inhibitory factors, application of growth factors is necessary to create the optimal environment for neuronal regeneration. Neurotrophins (neuro "relating to nerve cells"; trophe "nutrition") have consistently nourished the neuronal survival and sprouting effort in various models of spinal injury (Hulsebosch 2002; Jones et al. 2003). Zhou et al. (2003) showed that continued expression of neurotrophin-3 in the damaged corticospinal tract can improve axonal plasticity (Figure 2). Genetic induction of neurotrophin expression in fibroblasts, Schwann cells, olfactory ensheathing glia cells, and stem cells is a popular method of introducing growth-promoting grafts into the lesioned spinal cord. So far, viral vector-mediated neurotrophin gene transfer provides a less invasive form of regeneration induction than direct protein delivery (Hendriks et al. 2004). Using a gene delivery model with trophic support and a substrate for axonal growth, Blesch and Tuszynski (2003) implanted primary fibroblasts genetically modified ex vivo to secrete GDNF into mid-thoracic spinal cord transection sites. These GDNF grafts augmented considerable growth in dorsal column sensory, regional propriospinal, and local motor axons. Schwann cells were also induced to migrate to the transected site, spurring remyelination of damaged neurons (Maniwa et al. 2003). Additionally, axonal regeneration in rats with hemisectioned spinal cords was boosted by BDNF expressed in genetically altered fibroblasts. The rats were also rewarded with partial recovery of their forelimb function (Jin et al. 2002; Liu et al. 1999).
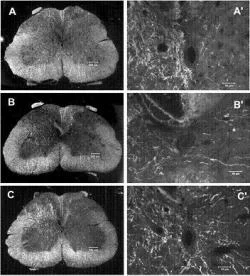
Figure 2. Corticospinal tract (CST) sprouting across the midline in the spinal cord after adenoviral vector neurotrophin 3 (Adv.EF -NT3) transduction of motoneurons. Dark-field photomicrographs of spinal cord cross sections showed the unlesioned CST axons. (A) Section from a normal rat (sham surgery). (B) Section from an Adv.EF -LacZ-treated rat (control vector). (C) Section from an Adv.EF -NT3-treated rat. CST neurites can be seen arising from the intact CST, traversing the midline, and growing into the grey matter of the lesioned side of the spinal cord. (A'-C') Higher-power photomicrographs of the regions around the central canal (Zhou et al. 2003).
A number of studies has shown that the effects of neurotrophin treatment are enhanced when utilized in combination with other regeneration therapies. In one such study by Lu et al. (2004), the soma of the L4 dorsal root ganglion of rat models were pre-conditioned with cAMP five days prior to lesion transection (previous research had shown that cAMP diminishes the inhibitory effects of myelin proteins if administered 1-7 days after injury). Neurotrophin-3 was injected within and beyond the spinal cord lesion site after injury. In a period of three months, rats treated with both cAMP and neurotrophin-3 exhibited long projections of sensory axons into and beyond the transected site, while rats receiving cAMP or neurotrophin-3 alone did not show axonal regeneration beyond the lesion site. Qiu et al. (2002) described an experiment in which axonal migration beyond the lesion was observed, but the results of both studies suggest that combination therapy can enhance the distance of axonal regeneration.
Tissue graft transplantation
Cellular transplantation is one of the most studied methods of repairing CNS tissue and will most likely be the first regeneration method to enter clinical trials. Grafts of fetal tissue, Schwann cell-containing peripheral nerves, Schwann cells, olfactory ensheathing cells (OECs), and recently, glial precursor cells have been demonstrated to bridge axons across the injury site and to enhance neuronal growth (Jones et al. 2003).
Current literature agrees that fetal tissue can survive in adult spinal cord and can allow host axons to integrate into the transplant. Some studies showed that the fusion of host and transplanted fetal cells was able to induce synapses and, in the midst of large lesions, even diminish gliosis around the transplant site (Bregman and Kunkel-Bagden 1988; Houle 1992; Privat et al. 1989; Tessler 1991). Few reports have shown much host axonal extension into the transplant tissue. However, combined treatment with neurotrophins BDNF and neurotrophin-3 has induced significant extension and branching in damaged axons (Bregman et al. 2002; Coumans et al. 2001).
Hill et al. (2004) completed a novel study of fetal tissue transplant via the glial-restricted precursor cell (GRP), which is capable of generating oligodendrocytes and two kinds of astrocytes in vitro. Rat models were injected with 500,000 embryonic GRPs directly into the lesion site. Transplantation down-regulated glial scarring and proteoglycan deposition throughout the six weeks of incubation without effecting uncontrolled glial production. The fine mesh observed around the lesion indicated immature astrocytes, which, along with the absence of proteoglycan, would make the lesion area more conducive for axonal regeneration.
Schwann cell-containing peripheral nerves have quite faithfully stimulated CNS cell regeneration (Qiu et al. 2002). Like in fetal grafts, damaged fibers that use peripheral nerves as the substrate for regeneration rarely extend far enough into the neural pathways needed for complete communication between cells. Levi et al. (2002) confirmed this point by showing that host axons of spinal-injured monkeys grew with peripheral nerve grafts but did not progress beyond the lesion sites. Levi's study was novel nevertheless in that it showed spinal injuries and graft therapy can be translated from the rat model to primates. Ten cynomologus monkeys underwent T-11 laminectomy and resection of hemispinal cord; after 4 months, the five that received the additional intercostal nerve grafts exhibited spinal axon regeneration, and immunostaining indicated remyelination around the resected area. Both graft and non-graft monkeys regained some hind limb function, although this result may be due to spontaneous recovery. Had the host axons extended further in the presence of peripheral nerves, more motor function might have been recovered.
Schwann cells of the peripheral nerve produce growth factors, cell adhesion molecules, and extracellular matrix components that enhance axonal regeneration. Remyelination of rat spinal cord when treated with cultured Schwann cells was observed, although with a lower density than intact spinal axons (Lankford et al. 2002; Xu et al. 1997). Help from neurotrophins and OECs may be required to improve axon fiber migration along the host-graft interface. BDNF and neurotrophin-3 induce positive chemokinesis of Schwann cells so that recruitment of Schwann cells near neurotrophin delivery sites can synergize the axonal growth process (Maniwa et al. 2003). Weidner et al. (1999) grafted Schwann cells induced to hyper-secrete human nerve growth factor (NGF) into spinal cord injury sites in adult rats. Those Schwann cells over-expressing NGF robustly increased axonal growth of the host tissue but did not enhance the extension of axonal length, compared to axons treated with Schwann cells alone. Nevertheless, Schwann cells can be grown in large amounts in vitro, implying that Schwann cells can be extracted from a peripheral nerve of a spinal-injured, cultured, and grafted back into the patient's own spinal cord a process likely to evade immune reactions.
Further research has found OECs to be the only glial cells capable of long axonal regeneration by folding the axons and guiding them as they extend into the hostile post-injury CNS environment (Ramon-Cueto et al. 1998). This discovery means that impaired axons can progress beyond the host-graft interface. In addition, OECs are pluripotent cells found in the olfactory bulb or the nasal olfactory mucosa. In fact, OECs from the latter area provide less invasive extraction, and studies have documented their functional restorative effects in rats after transplantation (Lu et al. 2001). Collection of OECs from the human nose, consequently, should not be a far-fetched idea. Meanwhile, OECs isolated from the olfactory bulb have remarkably allowed impulse conduction velocity in remyelinated host axons to nearly match that of normal axons. OECs can also simultaneously improve two spinal functions, as cervical-injured rats treated with OECs regained both major breathing and climbing behavior (Li et al. 2003). However, some scientists still question the reparative power of OECs and research on transplantation therapy must strive to find more strategies in promoting host axons to extend beyond the graft interface.
Stem cell therapy
Pluripotent stem cells can generate diverse types of cells and for this phenomenon, stem cell therapy has gained popularity as a treatment option for SCI. The three main sources of stem cells are derived from the brain, the bone marrow, and the embryo.
Neural stem cells can give rise to both neurons and glia, but so far these stem cells transplanted into contused rat spinal cords have only differentiated into astrocytes (no oligodendrocytes or neurons), with no functional recovery demonstrated (Kwon et al. 2002). However, when the adult rat spinal cord's own supply of stem cells is injected into the dentate gyrus, they can generate neurons, astrocytes, or oligodendrocytes, depending on the environmental cues (Jones et al. 2003). Human neural stem cells have been procured from the hippocampus, the ventricular/ependymal region, and the cortex, but very rarely from the spinal cord. Remarkably, scientific conjectures estimate around 500,000 to 2 million new cells, capable of becoming astrocytes and oligodendrocytes are born each day in the adult rat spinal cord one month after injury (Myckatyn 2004). This number offers guidance on the optimal number of cells for spinal repair in mammals. One seminal study by Akiyama et al. (2001) delineated the potential of human stem cells as treatment for SCI: adult human neural stem cells transplanted into the adult rat injured spinal cord demonstrated profuse axon myelination and electrical conduction at near normal speed (Figure 3). Thus, these neuroprogenitor cells (NPCs) from the subventricular zone exhibit some functional recovery. Fujiwara et al. (2004) looked for a less invasive method of stem cell therapy and found that NPCs derived from embryonal rat hippocampus, when injected intravenously, migrated to the spinal lesion site and lasted for 56 days while retaining the ability to differentiate into neurons, oligodendrocytes, and astrocytes. Thus, intravenous effusion will prove useful in designing stem cell applications for humans.
Figure 3. Remyelination of the rat spinal cord following transplantation of adult human precursor cells. Normal (A), demyelinated (B), and remyelinated axons (C) of the dorsal column. (D) Remyelinated axons at higher magnification. The anatomical pattern of myelination was similar to that produced by Schwann cells (arrows). (bar: AC, 25 µm; D, 10 µm) (Akiyama et al. 2001).
Bone marrow stem cells have also been shown to actively remyelinate spinal cords and can be administered directly or intravenously (Jones et al. 2003; Akiyama et al. 2002). Stem cells are unique in retaining function after intravenous application, while other myelinating factors like Schwann cell and OECs have been ineffective upon intravenous injection (Akiyama et al. 2002). Where transplanted peripheral nerves have failed to stimulate long axonal fibers, bone marrow stem cells directly delivered to the spinal cord lesion have formed bundles of neurofilament fibers between the host and graft tissue that guide regenerating axons to extend across the lesion. Hofstetter et al. (2002) demonstrated this process in the rat model, which also regained some locomotor function. The availability of bone marrow stem cells lay potential for autologous transplantation as treatment for SCI in the future.
The use of embryonic stem cells has sparked the most ethical controversy. Despite an initial burst of studies on their ability to differentiate into neurons and glial cells, remyelinate axons, and restore neural function, current research has been slow to follow up. Also embryonic stem cells have demonstrated a tendency for abnormal cell development and tumor formation in vivo (Jones et al. 2003). Indeed, cellular differentiation, in terms of both the genetic pathway and the environmental cues, needs to be painstakingly controlled should stem cell therapy for SCI be brought to clinical trials.
Clinical Outlook
Figure 4. Schematic diagram of a piece of spinal cord that has sustained an initial injury (black central oval) that spreads progressively outward and radially (red circular region followed by orange circular region) in zones until it finally reaches the final lesion size (grey shaded area). Blue lines are axons, and green rectangles are the myelinating oligodendrocytes. Methods of intervention: 1) reduction of edema and free radical production, 2) rescue of neural tissue at risk of dying in secondary processes such as abnormally high extracellular glutamate concentrations, 3) control of inflammation, 4) rescue of neuronal/glial populations at risk of continued apoptosis, 5) repair of demyelination and conduction deficits, 6) promotion of neurite growth through improved extracellular environment, 7) cell replacement therapies, 8) efforts to bridge the gap with transplantation approaches, 9) efforts to retrain and relearn motor tasks, 10) restoration of lost function by electrical stimulation, and 11) relief of chronic pain syndromes (Hulsebosch 2002).
For the treatment of SCI, McDonald and Sadowsky (2002) offer a hierarchy of interventions, with each successive need increasing in importance: 1) limiting secondary damage to spinal tissue, 2) remyelinating axons or compensating for it, 3) removing or abating inhibitory factors, 4) promoting axonal regeneration, 5) guiding axons to make the right connections, 6) creating bridges to connect fibers 7) replacing injured or dead cells. These seven major interventions exist for the purpose of optimizing neuronal regeneration and restoring neuronal function (Figure 4). The best clinical treatment should allow humans to regain both locomotor and physiological functions. An improvement in the quality of an SCI victim's life does not depend on complete restoration of the spinal cord. Instead, fair regeneration of axons can go a long way to restoring motor capability and emotional and psychological well-being.
Current clinical trials
Methods of neuronal regeneration, despite the research advances, are slow to enter clinical trials due to the need for success in primate models and consistent, solid evidence. Previous trials have mainly been pharmacological. Nevertheless, currently phase 1 human clinical trials have begun for private company, Diacrin's embryonic porcine neural stem cell implants and Proneuron's autologous macrophage transplants for inducing growth-permissive cellular environments (Jones et al. 2003). In addition, clinical trials for the treatment of paraplegia via nasal-derived OEC transplants began in 2002 (Jones et al. 2003). Clearly, neuronal regeneration strategies are still very much in a laboratory research boom.
Animal models
Several problems exist in using animal models for treatment of a human condition. First, the lesions induced by SCI in humans are likely to be much more complex than those experimentally inflicted in rat models. Each actual SCI case in the world features a different lesion, while lesions in the lab have to be made as reproducible as possible for consistent data. Rats also tend to experience a faster recovery from SCI than humans (Jones et al. 2003). Axonal regeneration in humans will have to reach longer distances, so some research touting noticeable axon sprouts in rat CNS may be insignificant in humans. Human models may facilitate the record of locomotor and physiological function after treatment, as communication is far advanced in human beings than in rats (Sipski 2003). Regardless, the rat is the closest model to humans and studies comparing a contusion in rat and human spinal cord showed parallel morphological, electrophysiological, and functional characteristics (Jones et al. 2003).
Issues in clinical trials
Why do so few clinical trials for new advances SCI treatment exist? Safety is the paramount concern. Since most studies report only partial axonal regeneration and restoration of function, and few experiments document the enhanced axon's ability to connect and communicate with other neurons, the questions of which spinal fibers will regenerate, how much will they extend, what connections will they make, and how will they interact with growth-promoting factors like neurotrophins or Nogo antibodies remain unanswered. Furthermore, human trials cannot tolerate severe side effects for largely emotional and sociopolitical reasons. Still other concerns are raised: which of the regeneration therapies should be tested first; which combination therapies are ideal; who should be treated first the chronically or acutely injured? A number of studies involving neurotrophins, transplants, and OECs have shown greater axonal regeneration and functional recovery when treatment was delayed for weeks after injury (Jones et al. 2003). The larger the time window of opportunity for therapy, the more practical the therapy is for humans. Although findings that human neurons retain their ability to regenerate 5 to 8 years post-injury are promising, the best treatment for SCI depends on a holistic analysis of all aspects of the injury, from the mechanism of injury to the cellular disruption to the functional loss.
Conclusion
The long-held belief that CNS tissue cannot regenerate is no longer valid. To overcome the physical and chemical barriers to neuronal growth, current research focuses on active cellular interventions as SCI therapy. Control of reactive astrocyte secretions, blockade of myelin-associated proteins, introduction of neurotrophins, and transplantation of special peripheral nerve and stem cells have been verified to encourage neuronal regeneration by creating a less hostile environment for growth. However, before scientists spearhead into clinical trials, more research must focus on axonal cross of the host-graft interface, minimally invasive transplantation therapy, restoration of physiological deficiencies in addition to locomotor function, and combination therapies for safe, controlled human trials. No panacea exists for SCI, but the boom in research advancements represents a creative fusion of the methods of immunology, developmental, cell, and molecular biology that points towards a promising translation of neuronal regeneration from the laboratory model to the ailing patient.
References
Akiyama Y. et al. (2001) Transplantation of clonal neural precursor cells derived from adult human brain establishes functional peripheral myelin in the rat spinal cord. Exp Neurolg. 167: 27-39.
Akiyama Y. et al. (2002) Remyelination of the spinal cord following intravenous delivery of bone marrow cells. Glia. 39: 229-236.
Blesch, A. and M. Tuszynski (2003) Cellular GDNF delivery promotes growth of motor and dorsal column sensory axons after partial and complete spinal cord transections and induces remyelination. J Comp Neurol. 467: 403-417.
Bomstein Y. et al. (2003) Features of skin-coincubated macrophages that promote recovery from spinal cord injury. J Neuroimmunolog. 142: 10-16.
Bracken M.B. et al. (1992) Methylprednisolone or naloxone treatment after acute spinal cord injury: 1-year follow up data. J Neurosurg. 76: 23-31.
Bradbury E.J. et al. (2002) Chondroitinase ABC promotes functional recovery after spinal cord injury. Nature. 416: 636-40.
Bregman B.S. and E. Kunkel-Bagden (1988) Effect of target and non-target transplants on neurons survival and axonal elongation after injury to the developing spinal cord. Prog Brain Res. 78: 205-211.
Bregman B.S. et al. (2002) Transplants and neurotrophic factors increase regeneration and recovery of function after spinal cord injury. Prog Brain Res. 137: 257-273.
Coumans J.V. et al. (2001) Axonal regeneration and functional recovery after complete spinal cord transaction in rats by delayed treatment with transplants and neurotrophins. J Neuroscience. 21: 9334-9344.
Fujiwara Y. et al. (2004) Intravenously injected neural progenitor cells of transgenic rats can migrate to the injured spinal cord and differentiate into neurons, astrocytes and oligodendrocytes. Neurosci Lett. 366: 287-291.
GrandPre S. et al. (2002) Nogo-66 receptor antagonist peptide promotes axonal regeneration. Nature. 417: 547-551.
Hendriks W.T. et al. (2004) Viral vector-mediated gene transfer of neurotrophins to promote regeneration of the injured spinal cord. Prog Brain Res. 146: 451-476.
Herman R. et al. (2002) Spinal cord stimulation facilitates functional walking in a chronic, incomplete spinal cord injury. Spinal Cord. 40: 65-68.
Hill C.E. et al. (2004) Acute transplantation of glial-restricted precursor cells into spinal cord contusion injuries: survival, differentiation, and effects on lesion environment and axonal regeneration. Exp Neurol. 190: 289-310.
Hofstetter C.P. et al. (2002) Marrow stromal cells form guiding strands in the injured spinal cord and promote recovery. Proc Natl Acad Sci. 99: 2199-2204.
Houle J.D. et al. (1992) The structural integrity of glial scar tissue associated with a chronic spinal cord lesion can be altered by transplanted fetal spinal cord tissue. J Neurosci Res. 31: 120-130.
Hulsebosch C.E. (2002) Recent advances in pathophysiology and treatment of spinal cord injury. Adv Physiol Educ. 26: 238-255.
Jin Y. et al. (2002) Transplants of fibroblasts genetically modified to express BDNF promote axonal regeneration from supraspinal neurons following chronic spinal cord injury. Exp Neurol. 177: 265-275.
Jones D.G. et al. (2003) Spinal cord regeneration: moving tentatively towards new perspectives. NeuroRehab. 18: 339-351.
Kwon B.K. et al. (2002) Survival and regeneration of rubrospinal neurons one year after spinal cord injury. Proc Natl Acad Sci. 99: 3246-3251.
Lankford K.L. et al. (2002) A quantitative morphometric analysis of rat spinal cord remyelination following transplantation of allogenic Schwann cells. J Comp Neurol. 443: 259-274.
Levi A.D. et al. (2002) Peripheral nerve grafts promoting central nervous system regeneration after spinal cord injury in the primate. J Neurosurg Spine. 96: 197-205.
Li S. et al. (2004) Blockade of Nogo-66, myelin-associated glycoprotein, and oligodendrocyte myelin glycoprotein by soluble Nogo-66 receptor promotes axonal sprouting and recovery after spinal injury. J Neuroscience. 24: 10511-10520.
Li Y. et al. (2003) Transplantation of olfactory ensheathing cells into spinal cord lesions restores breathing and climbing. J Neuroscience. 23: 727-731.
Liu Y. et al. (1999) Transplants of fibroblasts genetically modified to express BDNF promote regeneration of adult rat rubrospinal axons and recovery of forelimb function. J Neuroscience. 19: 4370-4387.
Lu J. et al. (2001) Transplantation of nasal olfactory tissue promotes partial recovery in paraplegic adult rats. Brain Res. 889: 344-357.
Lu P. et al. (2004) Combinatorial therapy with neurotrophins and cAMP promotes axonal regeneration beyond sites of spinal cord injury. J Neuroscience. 24: 6402-6409.
Maniwa S. et al. (2003) Effects of neurotrophic factors on chemikinesis of Schwann cells in culture. Scand J Plast Reconstr Surg Hand Sur. 37: 14-17.
McDonald J.W. and C. Sadowsky (2002) Spinal cord injury. Lancet. 359: 417-425.
Menet V. et al. (2004) Axonal plasticity and functional recovery after spinal cord injury in mice deficient in both glial fibrillary acidic protein and vimentin genes. Proc Natl Acad Sci. 100: 8999-9004.
Merkler G. et al. (2001) Locomoter recovery in spinal cord-injured rats treated with an antibody neutralizing the myelin-associated neurite growth inhibitor Nogo-A. J Neuroscience. 21: 3665-3673.
Myckatyn T.M. et al. (2004) Stem cell transplantation and other novel techniques for promoting recovery from spinal cord injury. Transp Immunolg. 12: 343-358.
Nieto-Sampedro M. (2003) Central nervous system lesions that can and those that cannot be repaired with the help of olfactory bulb ensheathing cell transplants. Neurochem Res. 28: 1659-1676.
Privat A. et al. (1989) Intraspinal transplants of serotonergic neurons in the adult rat. Brain Res Bull. 22: 123-129.
Qiu J. et al. (2002) Spinal axon regeneration induced by elevation of cyclic AMP. Neuron. 34: 895-903.
Ramon-Cueto A. et al. (1998) Long distance axonal regeneration in the transected adult rat spinal cord is promoted by olfactory ensheathing glia transplants. J Neuroscience. 18: 3803-3815.
Ribotta M.G. et al. (2004) Glial scar and axonal regeneration in the CNS: lessons from GFAP and vimentin transgenic mice. Acta Neurochir Suppl. 89: 87-92.
Sadowsky C. et al. (2002) Spinal cord injury. Disab Rehab. 24: 680-687.
Simonen M. et al. (2003) Systemic Deletion of the Myelin-Associated Outgrowth Inhibitor Nogo-A Improves Regenerative and Plastic Responses after Spinal Cord Injury. Neuron. 38: 201-211.
Sipski M.L. (2003) From the bench to the body: key issues associated with research aimed at a cure for SCI. J Rehab Res Dev. 40(4 Suppl 1): 1-7.
Tessler A. (1991) Intraspinal transplants. Ann Neurol. 29: 115-123.
Weidner N. et al. (1999) Nerve growth factor-hypersecreting Schwann cell grafts augment and guide spinal cord axonal growth and remyelinate central nervous system axons in a phenotypically appropriate manner that correlates with expression of L1. J Comp Neurol. 413: 495-506.
Xu X.M. et al. (1997) Bridging Schwann cell transplants promote axonal regeneration from both the rostral and caudal stumps of transected adult rat spinal cord. J Neurocytolog. 26: 1-16.
Zhou J. et al. (2003) Neurotrophin-3 expressed in situ induces plasticity in the adult injured spinal cord. J Neuroscience. 23: 1424-1431.